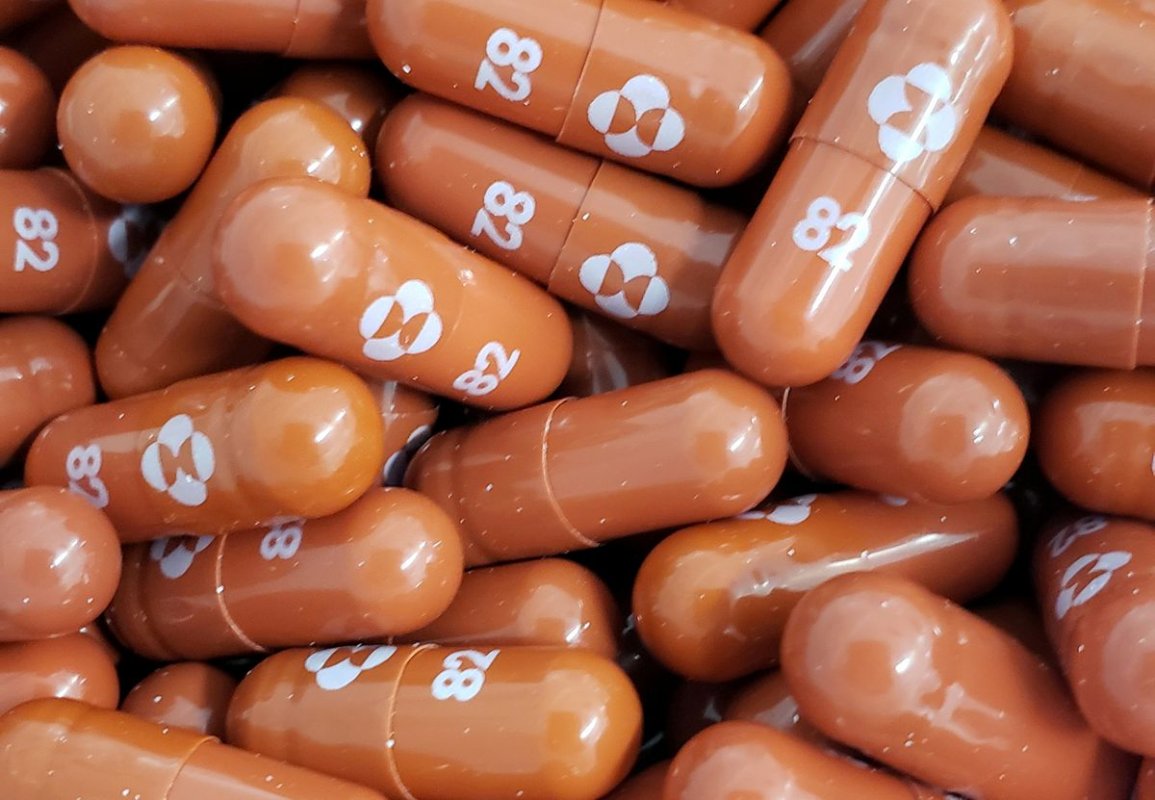
Last week Britain became the first country in the world to approve the potentially game-changing COVID-19 antiviral pill, jointly developed by U.S.-based Merck & Co Inc and Ridgeback Biotherapeutics.
The government said in October it had secured 480,000 courses of the Merck drug, as well as 250,000 courses of an antiviral pill developed by Pfizer Inc (PFE.N).
Asked about the molnupiravir approval, Hopkins told BBC television: "That is great news and it will start to be rolled out through a drug trial at the end of this month/the beginning of December."
Hopkins said all the trials so far had been done with the unvaccinated, so this would help understand how it will work in the wider vaccinated population.
"The new Pfizer drug is probably not going to be licensed until the new year sometime," she added. "It is still likely to be a couple of months away."
資料來源:https://www.reuters.com/business/healthcare-pharmaceuticals/uk-roll-out-covid-19-antiviral-drug-trial-this-month-health-security-agency-2021-11-07/
(2021年11月10日)
上述資料僅供參考。世紀21奇豐國際沒有就該等資料的準確性或完整性,或在任何特定情況下使用的合適性作出任何明示或隱含的保證,亦無須對與該等資料或本網站有關的任何原故而引致的任何損失或損害負上責任。如有需要,請向相關部門或機構。